In recent years, the landscape of continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BiPAP) machines has undergone significant turbulence. One pivotal moment in this journey was Philips’ recall of these essential respiratory devices in 2021, sending shockwaves throughout the healthcare industry. This recall, coupled with various other factors, has contributed to an ongoing shortage of CPAP and BiPAP machines, affecting countless individuals in need of these critical medical devices.
The Philips Recall: A Turning Point:
Philips, a renowned name in the world of medical equipment, issued a recall of CPAP, BiPAP, and ventilator machines in 2021. This recall was prompted by concerns over potential health risks associated with the sound abatement foam used in these devices. While the intention was to ensure patient safety, the recall had far-reaching consequences.
By May 2023, Philips had successfully manufactured approximately 98% of the needed replacements. However, despite this significant progress, the situation remained challenging due to lingering distribution issues. These challenges have left many individuals without the essential respiratory support they require.
Chip Shortages and Supply Chain Disruptions
- Factors Exacerbating the Shortage:
The shortage of CPAP and BiPAP machines cannot be solely attributed to the Philips recall. One of the most prominent factors amplifying the crisis is the ongoing global chip shortage and supply chain disruptions. These issues have affected various industries, including healthcare, causing delays in production and distribution. - Defective Replacements:
In addition to the supply chain challenges, some of the replacements produced by Philips have been found to have defects. These defects necessitated another round of recalls in April 2023, further exacerbating the shortage. The recall of refurbished machines added to the complexity of the situation, leaving healthcare providers and patients grappling with uncertainty. - Increased Demand:
The increased demand for CPAP and BiPAP machines is another critical aspect contributing to the shortage. The growing awareness of sleep apnea and related respiratory conditions has led to more individuals seeking diagnosis and treatment. This surge in demand has put additional pressure on manufacturers to meet the needs of patients.
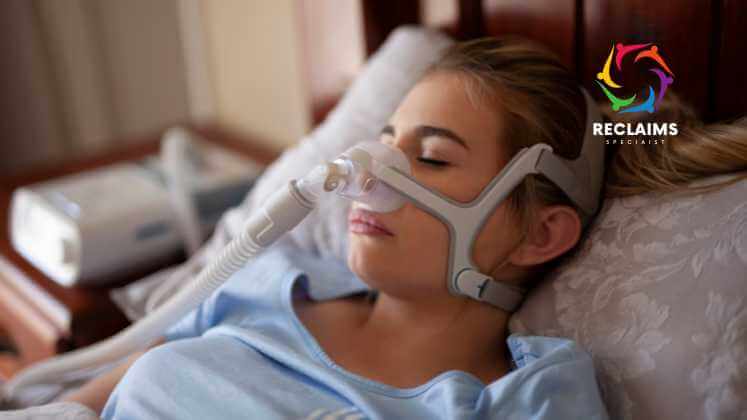
ResMed: Stepping In to Bridge the Gap
Amidst the shortage crisis, ResMed, a prominent competitor in the respiratory device market, has taken steps to fill the gap. The company reported record profits and significantly expanded its production capacity to help address the soaring demand for CPAP and BiPAP machines. ResMed’s proactive approach has played a pivotal role in providing relief to those affected by the shortage.
What You Can Do
If you are among the individuals in need of a CPAP or BiPAP machine, there are steps you can take to navigate this challenging situation:
- Contact Philips: If you were using a Philips CPAP or BiPAP machine affected by the recall, get in touch with the company. They should be able to provide you with information on the status of your replacement and guide you through the process.
- Consult Your Medical Provider: Discuss your options with your healthcare provider. They can assess your specific needs and recommend alternative solutions or devices from reputable manufacturers.
- Stay Informed: Keep yourself informed about the latest developments in the CPAP and BiPAP shortage. As the situation evolves, new solutions and resources may become available.
Legal Matters and Patient Safety:
It’s worth noting that lawsuits have arisen in the wake of the CPAP and BiPAP machine recall. Philips has maintained that emissions from the foam in their devices are unlikely to harm patients. However, it is essential for individuals affected by the recall to consult legal counsel if they have concerns about their health and safety.
In conclusion, the shortage of CPAP and BiPAP machines has been a complex and challenging issue, with multiple factors converging to create a significant impact. While progress has been made in addressing the shortage, it remains essential for individuals affected to stay informed, seek guidance from medical professionals, and explore available options. The resilience of the healthcare industry and the proactive measures of companies like ResMed offer hope for a brighter future in ensuring access to vital respiratory equipment.
If you have been in Camp Lejeune Or Used Talcum Powder or Affected by Defected Hernia mesh Or any other product causing harm to you fill the form below and we will guide you to your compensation money for free.


